NuVasive Magec System Scoliosis Rods
Neumann Law Group Reviewing Product Liability Cases Related to Magec System Scoliosis Rods
The Neumann Law Group is looking into claims against NuVasive related to their defective medical device designed to treat scoliosis in children, the Magec System. Scoliosis, an abnormal curvature of the spine, is a common condition in both adults and adolescents. Children with a diagnosis prior to the age of 10, known as early-onset scoliosis (“EOS”), have a much higher risk of developing significant complications including poor heart and lung function. Often, they will be placed into braces or casts for treatment, although unfortunately at times surgery is necessary.
Prosthetic ribs, vertebral tethering, and spinal fusion are examples of the many surgical methods that have been used to treat early-onset scoliosis over the years. One of the most common options is to resize and replace the growth rods with surgery every six months. Looking for an option which would keep the children from needing repeated surgeries, NuVasive made a product called the Magec System. This system allows doctors to use magnets to adjust the implanted growth rods versus requiring repeated surgery.
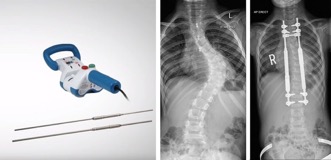
Where did the Magec System Go Wrong?
Despite the goal to keep the children out of surgery, in February 2020, NuVasive had to make a recall of the NuVasive Magec System Model X rods. They identified structural defects in the rods, mainly with ‘endcap separation’ resulting in the inner components being exposed to living tissue. The FDA cleared an updated version of the rod in July 2020 following efforts made by NuVasive to correct these problems. Still, NuVasive issued another Safety Notice in December 2020, this time in Europe.
Another warning came in April 2015. NuVasive acknowledged that due to substandard testing of the Magec System, ‘biocompatibility’ problems existed. This means that they did not even know whether the endcap material on the Magec System rod was compatible with human tissue. In July 2015, the FDA issued yet another warning about the Magec System rods, this time with concerns for:
- Metal Particles Shedding into Bloodstream
- Failure of the Rod to Lengthen (known as “Loss of Distraction”)
- Rod Breakage
- Rod Bending
- Endcap Failure
- Fractured Drive Pin
- Screw Breakage
- Locking Pin Breakage
- O-Ring Seal Failure
The claims against NuVasive Magec System currently allege that the product has been sold with knowledge of defects in design, manufacturing, and marketing defects.
The following serious complications—and more which are being identified—have been linked to these failures:
- Metallosis—blood poisoning from high levels of toxic metal in the blood
- Necrosis
- Tissue Discoloration
- Painful and unplanned revision surgery
These models are affected:
- Magec Spinal Bracing and Distraction System
- Magec 2 Spinal Bracing and Distraction System
- Magec System
- Magec System Model X device
- Magec System Model X rod
- Magec System Rods
NuVasive Prior Knowledge of Defects in Magec System
When a medical device such as the Macec System rod does not perform as advertised and the manufacturer becomes aware of the dangers, they have a responsibility to take action to correct the concerns or to issue recalls and prevent others from injury. Not only does NuVasive have the responsibility to take steps to repair the defective product, but they also have the responsibility to keep consumers informed about the dangers of their products. After correcting the problems following the initial recall in February 2020, NuVasive failed to warn consumers of the later problems with this device and did not issue any recalls. To date, NuVasive has not done any recalls or corrective actions related to this defective and dangerous product designed to treat Early Onset Scoliosis in young children.
As a result, claims against NuVasive Magec System allege that their product with sold with knowledge of defects in design, manufacturing, and marketing defects.
Who may be Eligible to Bring a Claim Against NuVasive?
You may be eligible to file a NuVasive Magec lawsuit if your child:
- Has an adjustable MAGEC System spinal rod implant
- Was 10 years old or younger when device was implanted
- MAGEC Rod was implanted on or after September 1, 2014
Our children and their doctors have put trust in companies like NuVasive and their Magec system to provide relief for the debilitating condition of early-onset scoliosis.
Schedule a Free Consultation with Neumann Law Group’s Experienced Defective Medical Device Attorneys in Traverse City, Grand Rapids or Detroit
When our children seek relief from a debilitating condition, we never expect that they will be injured by a medical device. If your family finds itself in this challenging situation, Neumann Law Group may be able to help you. Detroit, Grand Rapids, and Grand Rapids attorney Kelly Neumann can represent you in claims out of Petoskey, Warren, Holland, Midland, Muskegon, Saginaw, Wyoming, Kalamazoo, Lansing, Flint, Ann Arbor, and communities throughout the Upper Peninsula. Contact us at 800-525-NEUMANN or via our online form for a free consultation with a product liability attorney. We also represent victims in California and Massachusetts.







